Principal Investigator: Michael Sadowsky
Organization(s): University of Minnesota, Department of Soil, Water, and Climate
Award Amount: $240,000
Start Date: 3/1/2007 | End Date: 6/30/2009
Project Manager(s): Adam Birr
Development of DNA Marker Gene Systems for E.coli from Cows, Pigs, and Turkeys: Part 1 (PDF)
Development of DNA Marker Gene Systems for E.coli from Cows, Pigs, and Turkeys: Part 2 (PDF)
Background
 Fecal loading of aquatic environments by animals is of concern for public and environmental health. The deposition of fecal bacteria into waterways is thought to be one of the main routes by which humans are exposed to pathogens. The microbiological contamination of waterways is amongst the most commonly listed water quality impairment in the U.S. (USEPA, 2005).
Fecal loading of aquatic environments by animals is of concern for public and environmental health. The deposition of fecal bacteria into waterways is thought to be one of the main routes by which humans are exposed to pathogens. The microbiological contamination of waterways is amongst the most commonly listed water quality impairment in the U.S. (USEPA, 2005).
When any impairment occurs, states must conduct a Total Maximum Daily Load (TMDL) assessment in order to mitigate the impairment and restore the water body to acceptable quality. A critical step in this process is to identify the source(s) of bacteria and implement management practices that reduce pollutant loading.
In the first part of the study, researchers utilized subtraction suppressive hybridization (SSH) to identify DNAs that are specific for Escherichia coli (E. coli) originating from cows, pigs, and turkeys. Also, they were evaluating and using DNA PCR primers that are specific for fecal bacteria (members of the order Bacteroidales) originating from humans and cows. These host specific markers were used in the second part of the study to determine the source of fecal contamination on a watershed scale.
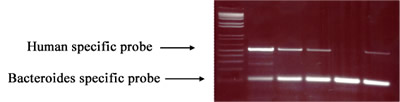
In part two of this study, researchers assessed the spatial and temporal dynamics of bovine fecal loading to a small creek system in southeastern Minnesota, the Little Jordan Creek.
The purpose of the second part of this study was to:
- Determine the scale and source of fecal loading to the creek in 2008
- Subsequently employ best management practices (BMPs) at the beginning of and throughout the 2009 sampling season to mitigate fecal loading to the system
Methods
Field investigations were performed on the Little Jordan Creek, near Chatfield, Minnesota. Five sites were sampled in 2008 and 2009. These sites included areas of the creek that were thought to be significantly impacted by grazing practices, and areas thought to have less cattle impact.
Two different molecular techniques were used to determine the presence and concentration of E.coli and fecal coliform within a specific watershed. For a more detailed discussion of the quantitative PCR protocol and plate count analysis refer to part two of the research report (PDF).
Results from the Little Jordan Watershed
- Timing of major rain events, proximity of cows to the creek, and water temperature were major factors influencing the number of E.coliand fecal coliforms.
- In 2009, the proximity of the cows to the stream and the timing of rain events proved to be the largest factors contributing to fluctuations of numbers of fecal coliforms and E.coli. This is perhaps due to the runoff carrying additional fecal materials to sections of the stream that would regularly not be influenced, combined with increased flow disturbing sediment and liberating bacteria that would normally not be identified by the water sampling technique used.
- Total fecal coliform counts were lowest during early sampling periods (April). This was likely due to several factors, including low water temperatures and low initial influence of cow manure in immediate upland areas. In addition, during this early part of the season animals were not given direct access to the stream corridor.
- E.coli numbers often exceeded the Minnesota State Standard of 126 CFU/100ml at several sampling locations spanning the entire creek, while highest levels were observed where the creek left the property.
Deliverables
- Identify DNA markers that are specific for E.coli strains isolated from cows, pigs, and turkey with quantifiable selectivity and sensitivity
- Perform field studies on the use of the developed DNA markers to determine sources of fecal contamination in a small watershed
- Evaluate the effectiveness of BMPs designed to mitigate fecal bacteria contamination from livestock sources

