- Ornamentals and golf course turf
- Greenhouse and nursery production
- Cattle (under some circumstances)
- Structures for humans and livestock/poultry
- Roach bait, and ant mounds
- Agricultural crops (conditional registration, see Chlorpyrifos Updates for details)
Most chlorpyrifos products are classified as a restricted use pesticides (RUP). Only licensed (commercial or non-commercial applicators) or certified (private applicators) can sell, buy, or use RUPs.
Chlorpyrifos has been detected in surface waters in Minnesota through the MDA's Ambient Water Monitoring Program and is currently designated as a "Surface Water Pesticide of Concern." Select water bodies in the state have also been listed as "impaired" due to elevated detections of chlorpyrifos. The MDA has developed Water Quality Best Management Practices for Chlorpyrifos (PDF) and increased education and outreach efforts.
How it Works
Chlorpyrifos acts as a nerve agent and is classified as an acetylcholinesterase (AChE) inhibitor (Group 1B) by the Insecticide Resistance Action Committee. Insects can be exposed to chlorpyrifos through either direct contact, ingestion, or inhalation.
Chlorpyrifos functions by binding to AChE, thereby preventing the breakdown of acetylcholine (a neural signal carrier). Subsequent accumulation of acetylcholine causes overstimulation of nerves which can result in paralysis, seizures, and eventual death of the insect. Other organophosphate insecticides that share this mode of action include diazinon, malathion, parathion, dichlorvos, and terbufos.
Use in Minnesota
As of February 2024, 7 products with chlorpyrifos as an active ingredient are registered in Minnesota.
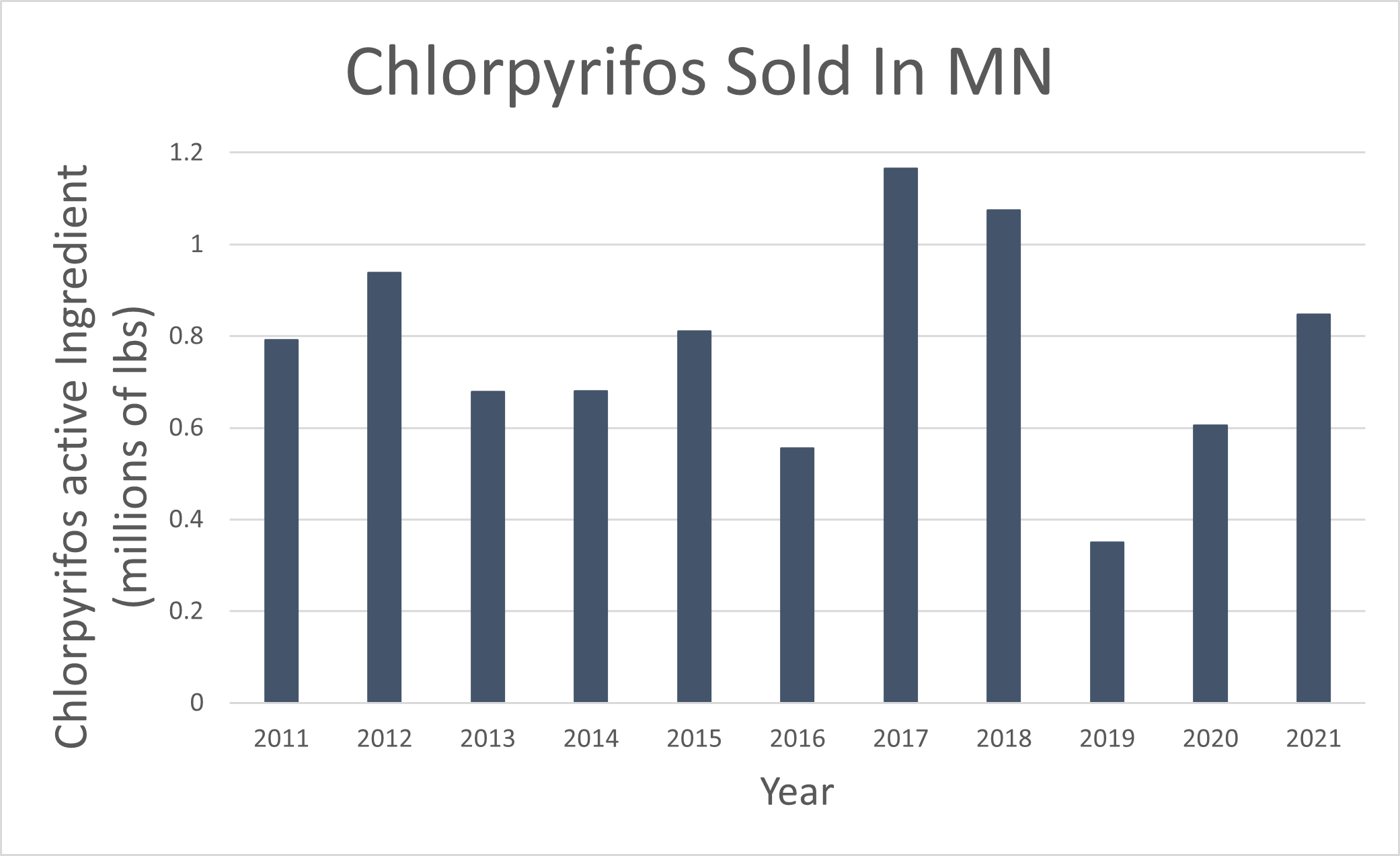
Historically, chlorpyrifos was the highest selling insecticide in the state with consistently high sales from 2011-2021 (see figure). The MDA gathers sales data from pesticide dealers and registrants to determine the number of pounds of pesticide products sold each year. Historic sales data can be found using the Pesticide Sales Database Search.
Chlorpyrifos in the Environment
Chlorpyrifos enters the environment from routine use where it can move and degrade in a number of ways. How chlorpyrifos moves is largely determined by its physical and chemical properties, as is the length of time it persists after application. Site-specific conditions, such as weather and soil type, are also key in determining its fate, along with the formulation applied (e.g., liquid vs. granular), method of application (e.g., ground-based or aerial), and other use factors (e.g., rate, timing). Chlorpyrifos has a low solubility in water and binds strongly to soil, which limits its mobility. Being relatively immobile in soil, leaching to groundwater is not expected; however, there is potential for chlorpyrifos to move with runoff into surface water, primarily with eroded sediment. Spray drift and volatilization from ground and aerial spray applications are additional ways chlorpyrifos can enter the environment. Spray drift can occur during the application process as fine spray droplets move away from the treated area. Volatilization of chlorpyrifos predominantly occurs from leaf surfaces shortly after foliar application. The resulting vapors can disperse and move offsite through atmospheric transport.
In soil, chlorpyrifos is primarily broken down through microbial degradation and its half-life can range from a few days to over 100 days. In aquatic environments, chlorpyrifos can undergo abiotic hydrolysis, biodegradation, and photodegradation. Oxidation and photolysis are key degradation processes in air and on foliar surfaces.
The major degradate of chlorpyrifos is 3,5,6-trichloro-2-pyridinol (TCP). TCP is mobile and persistent in soil (if not exposed to light). Chlorpyrifos can also undergo transformation to chlorpyrifos oxon, a minor degradate, under certain conditions. This degradate is less persistent than the parent, but more mobile in soil (Koc = 146-270 mL/goc). Chlorpyrifos oxon can also form as a byproduct of drinking water chlorination.
Human Health Risks
According to the U.S. Environmental Protection Agency (EPA), chlorpyrifos is moderately toxic to humans by oral, skin, and inhalation exposure, and not likely carcinogenic to humans [1]. Chlorpyrifos is metabolized in the human body to the more toxic and potent AChE inhibitor, chlorpyrifos oxon. Two toxicological endpoints, AChE inhibition and neurodevelopmental effects, have been used by the EPA for hazard characterization of chlorpyrifos and its oxon. Accumulation of acetylcholine in neural junctions due to the inhibition of AChE by chlorpyrifos oxon results in the acute signs and symptoms of cholinergic overstimulation (e.g., cramps, muscular weakness, paralysis, death).
Due to the potential risk to human health, the EPA has developed a set of requirements that must be followed when applying chlorpyrifos products.
Ecological Risks
According to the EPA’s Ecological Risk Assessment, chlorpyrifos is very highly toxic to birds and fish, highly toxic to honeybees, and moderately toxic to mammals [2].
Terrestrial organisms such as mammals, birds, and invertebrates may be exposed to chlorpyrifos and its degradates through multiple routes including contact, inhalation, and diet.
Aquatic organisms, including freshwater fish, invertebrates, and plants, can be exposed to chlorpyrifos or its degradates through contaminated surface water bodies such as lakes, ponds, rivers, and streams. Chlorpyrifos may bioaccumulate in organisms, which poses additional risk to organisms higher in the food chain. Due to toxicity to aquatic life, setbacks from surface water are required for many liquid product applications.
Chlorpyrifos toxicity values for aquatic and terrestrial organisms (Data from EPA [2] )
| Aquatic Organism | Toxicity Values | Toxicity Level |
|---|---|---|
| Freshwater fish | Acute LC50 a= 0.9 µg/L Reproductive NOAECb = 0.57 µg/L |
Very Highly Toxic |
| Freshwater invertebrates | Acute EC50c = 0.1 µg/L Reproductive NOAEC = 0.04 µg/L |
Very Highly Toxic |
| Terrestrial Organism | Toxicity Values | Toxicity Level |
|---|---|---|
| Birds | Acute LD50 d= 5.6 mg/kg Chronic NOAEC = 25 mg/kg |
Very Highly Toxic |
| Bees | Acute LD50 = 0.059 µg/bee | Very Highly Toxic |
| Mammals | Acute contact LD50 = 97 mg/kg Chronic NOAEC = 10 ppm |
Moderately Toxic |
a LC50 (lethal concentration) is the concentration of pesticide in water which kills 50% of a test population.
b NOAEC (no-observed-adverse-effects concentration) is the highest concentration of a toxicant which causes no detectable adverse effects on the test animals.
c EC50 (effective concentration) is the concentration of pesticide in water that causes an effect (e.g., immobilization) in 50% of a test population.
d LD50 (lethal dose) is the dose of pesticide which kills 50% of a test population.
References
- U.S. Environmental Protection Agency (USEPA). 2016. Chlorpyrifos: Revised human health risk assessment for registration review. EPA-HQ-OPP-2016-0062-0052.
- U.S. Environmental Protection Agency (USEPA). 2008. Problem Formulation for the Environmental Fate and Ecological Risk, Endangered Species and Drinking Water Assessments in Support of the Registration Review of Chlorpyrifos.

